Atoms are the building blocks of matter. All stars, planets, nebulae and galaxies are composed of atoms, and it is by understanding their basic structure that we can begin piecing together a picture of the way that stars emit light and spectra are produced.
The two main components of an atom are its nucleus and electron cloud. The nucleus is composed of both positively charged protons and electrically neutral neutrons. (Actually, the protons and neutrons themselves are made up of more basic particles called quarks; but protons and neutrons are as far as we need to get into the basic structure of an atom for this discussion). Surrounding the nucleus is a cloud of negatively charged electrons whose charges have the same magnitude as proton charges, but the opposite sign.
Learning highlight
According to quantum physics, electrons cannot be thought of as tiny particles orbiting around a nucleus. One of the fundamental principles of quantum physics, called the Heisenberg uncertainty principle, says that a particle’s velocity and position are not simultaneously knowable. The electron is therefore commonly thought of as a cloud, or orbital, surrounding the nucleus.
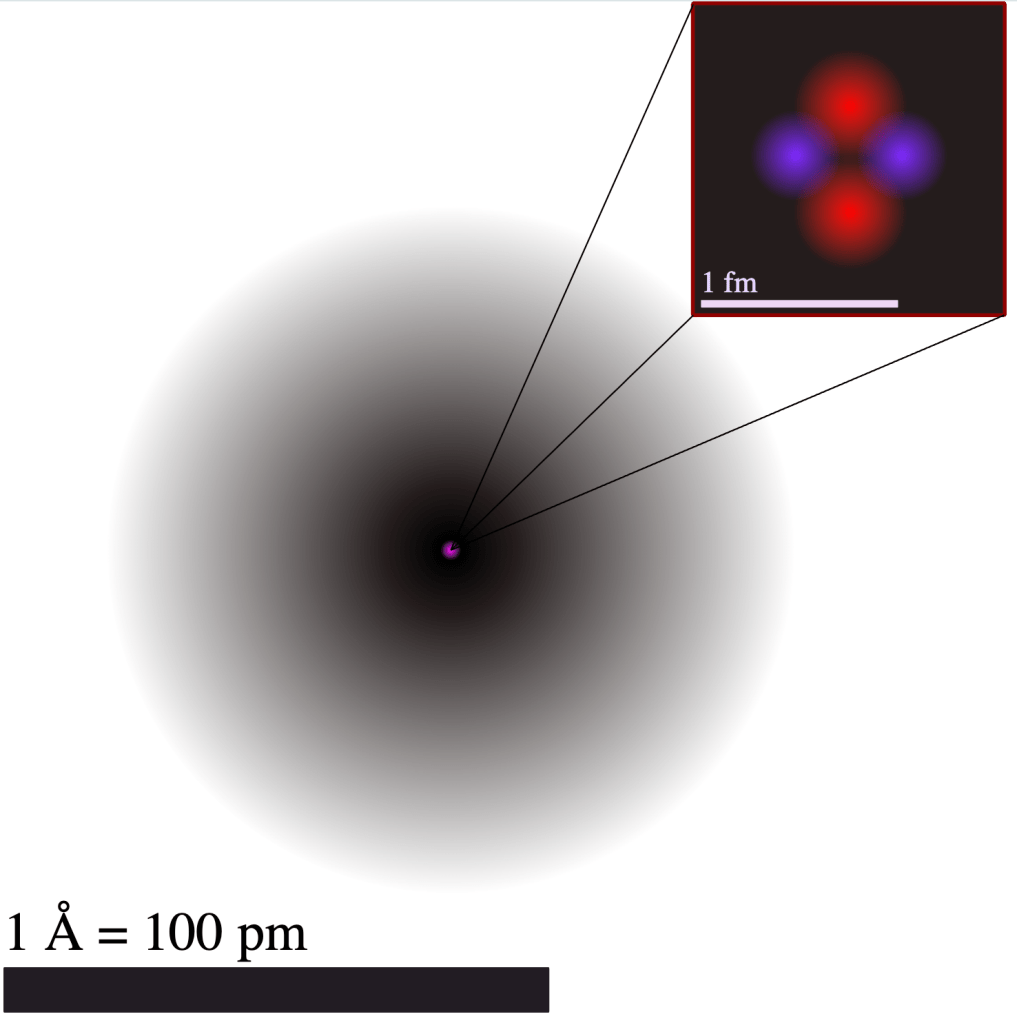
As noted in Module 4, an atom is mostly empty space. Figure 5-1 shows a diagram of a helium atom in which the electron cloud is roughly 100,000 times larger than the nucleus. For comparison, if the radius of the electron orbital were blown up to the size of a house this would mean that the nucleus at the centre would have the thickness of a piece of paper.
The number of protons in an atom determines the type of element it is. A single proton is a hydrogen atom, there are two protons in the nucleus of a helium atom, oxygen has eight protons, etc.
While the number of protons in an atom strictly determines the type of element, two atoms of the same element can actually have different numbers of neutrons. Similarly, two different elements can actually have the same number of neutrons. For example, the atom with one proton and one neutron, called deuterium, is a type of hydrogen. Tritium is another type of hydrogen atom with one proton and two neutrons. However, helium, with two protons and two neutrons (the same as tritium), is a different element all together.
Atoms with the same number of protons, but different numbers of neutrons are called isotopes of the same element.
If an atom has the same number of electrons and protons, it is overall electrically neutral since the charges of these particles are opposite. Electrons can be lost or gained by an atom, giving it an overall positive or negative charge (positive if electrons are lost, negative if they are gained). When this occurs, the atom is said to be ionised.
Learning Activity
In this brief summary, there are a lot of definitions of different particles and ways that their numbers differentiate the types of atoms that exist. You need to know them before going on to explore the different ways that light and matter interact. This can be done by taking a moment to list
- the particles defined
- their locations in an atom
- their charges, and
- which ones determine
- the element
- the isotope, and
- whether the atom is ionised.
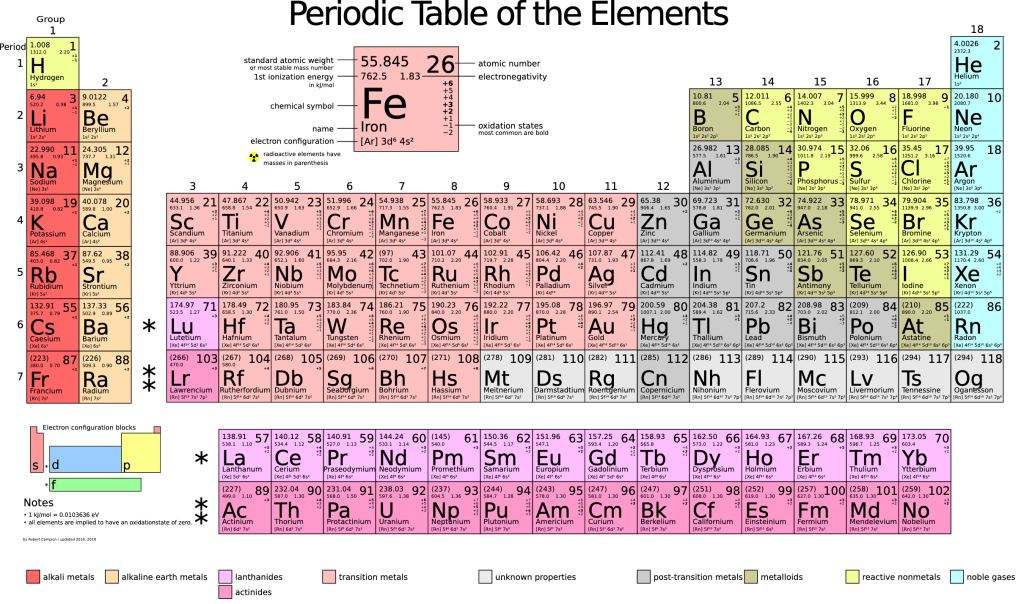
Now examine the periodic table of elements shown in Figure 5-2. What does the number in the top-right of each box correspond to? If an electron has much, much less mass than a proton or neutron, which have roughly equal mass, what determines the value in the top-left? The values shown in this table correspond to the most common isotopes of each element. How many neutrons are there in the most common isotope of Beryllium, the element with four protons? Answers: number of protons; combined masses of protons and neutrons; five.